
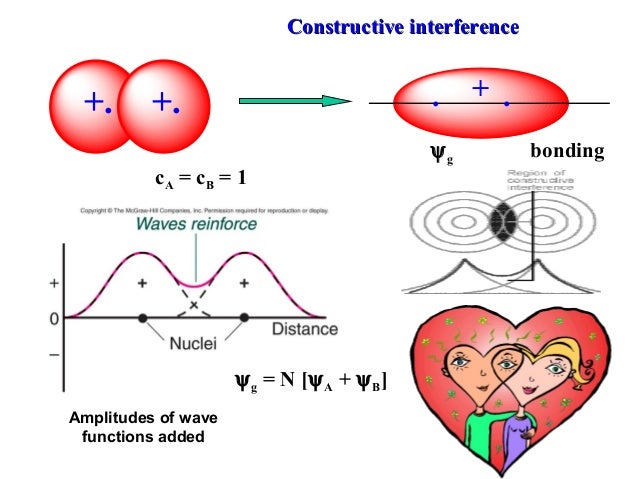
Increasing energy in case of the diatomic homonuclear Įnergy levels of these molecular orbitals have beenĭetermined experimentally by spectroscopic studies.The order of S 2s, s 2s, P 2p x, P * 2p x , P 2p y, P * 2p y , s 2p z, s 2p z. In the same manner the 2s and three 2p-orbitals of eachĪtom i.e., eight atomic orbitals can give rise to eight new Molecular orbitals designated as s 1s and s * 1s. Two 1s atomic orbitals of participating atoms give rise to two new In case of homonuclear diatomic molecules, combination of Singly filled with electrons having parallel spins. (ix) While filling molecular orbitals of equal energy, pairing ofĮlectrons does not take place until all such molecular orbitals are (viii) A molecular orbital can accommodate only two electrons and these Their energies, starting with orbital of least energy. (vii) The molecular orbitals are filled in the increasing order of (vi) The bonding molecular orbitals are represented by s (sigma), p (pi), d (delta) and the antibonding molecular orbitals are represented by s *, p*, d *. (v) The shapes of molecular orbitals depend upon the shapes With higher energy is called anti bonding molecular orbital. The molecular orbital with lower energy isĬalled bonding molecular orbita l and the other These two molecular orbitals one has a lower energy and the other has a (iv) Two atomic orbitals can combine to form two molecular orbitals.

(iii) The number of molecular orbitals formed is equal to the number of (ii) Molecular orbitals are formed by combination ofĪtomic orbitals of equal energies (in case of homonuclear molecules) or ofĬomparable energies (in case of heteronuclear molecules). (i) In a molecule, electrons are present in new orbitals called Molecules are present in new orbitals called molecular orbitals. Molecules, atomic orbitals lose their identity and the electrons in Molecular orbital theory was put forward by Hund and Molecular Orbital Theory : Energy level diagram for molecular


 0 kommentar(er)
0 kommentar(er)
